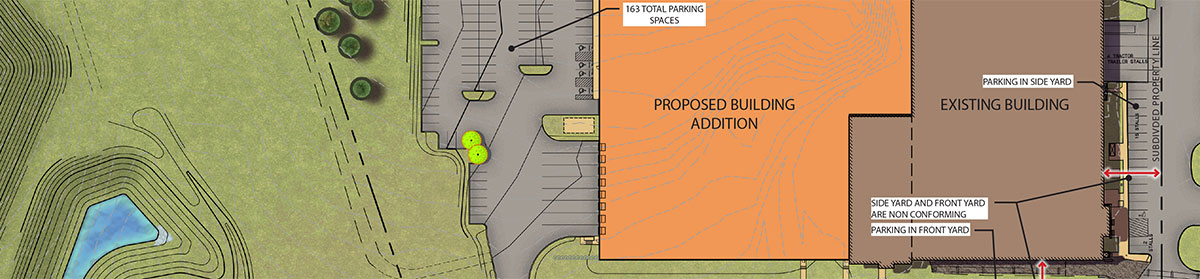
Major additions in Pennsylvania and Texas will meet increasing demand

From left to right; Carlos Dias – Senior VP, Global Operations, Technimark; John Rugari – VP Sales, Healthcare, Technimark; Brad Wellington – President & CEO, Technimark; Rosie Wolford – Mayor of Latrobe, PA; Victor Nazario – Director of Operations, Latrobe, Technimark